Olympus Confocal Laser Scanning Microscope FV4000
PT Wadya Prima Mulia as the Exclusive Distributor for Evident-Olympus in Indonesia provides Olympus Confocal Laser Scanning Microscope FV4000 and other products.
Transforming Precision Imaging
Empower Your Confocal Microscopy Imaging Experiments
Transform your images with the new FLUOVIEW™ FV4000 confocal laser scanning microscope. Advanced imaging technology enables higher precision images to empower researchers with more reliable data from their samples. With our breakthrough SilVIR™ detector at the core of the system, achieve much lower noise, higher sensitivity, and improved photon resolving capabilities. With the FV4000 confocal microscope, researchers can acquire higher-quality, quantitative image data in less time and with less effort.
For more information about the product click here
Experience the systems innovations, including:
- Game-changing dynamic range for imaging from the macro scale to subcellular structures
- Multiplex up to six channels simultaneously with TruSpectral™ technology
- Redesigned high-speed, high-resolution scanners for fixed and live cell imaging
- Improved depth and photosensitivity with pioneering near-infrared (NIR) capabilities and renowned optics
- Peace of mind with the reliable, repeatable SilVIR detector
- Industry-leading* ten laser lines with a broader spectral range from 405 nm to 785 nm
- Modular design to meet researchers’ ever-changing needs, including the ability to upgrade to multiphoton imaging on a single system
*As of October 2023.
Easy-to-Acquire, Quantitative Confocal Data
The FV4000 confocal microscope uses our advanced, silicon-based SilVIR™ detector that makes it easier than ever to acquire precise, reproducible data.
SilVIR Next-Generation Detector Technology
The SilVIR detector combines two advanced technologies—a silicon photomultiplier (SiPM) and our patented* fast signal processing design.
- High dynamic range
- Low noise
- No degradation of sensitivity
- Less sensitivity variation among other detectors
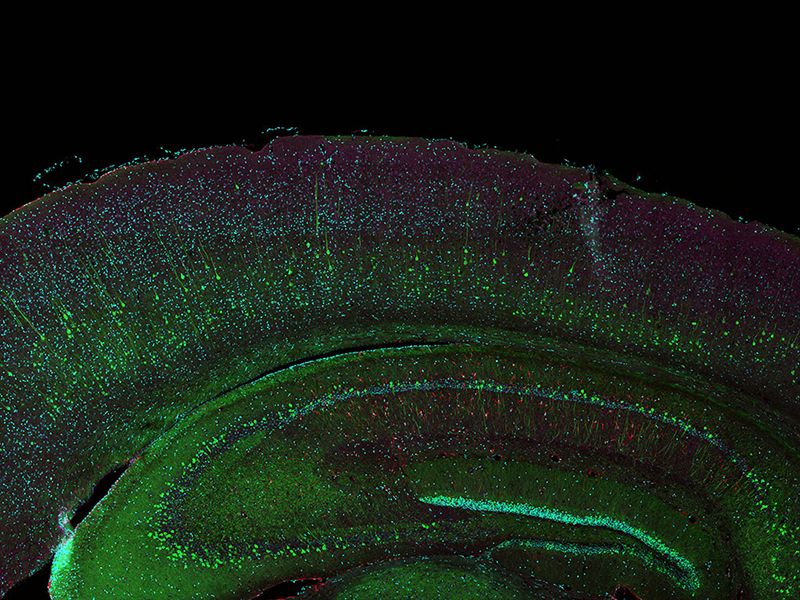
Sample courtesy of Katherine Given, Ph.D. Principal Investigator, Neurobiology University of Colorado Anschutz Medical Campus, Aurora, Colorado
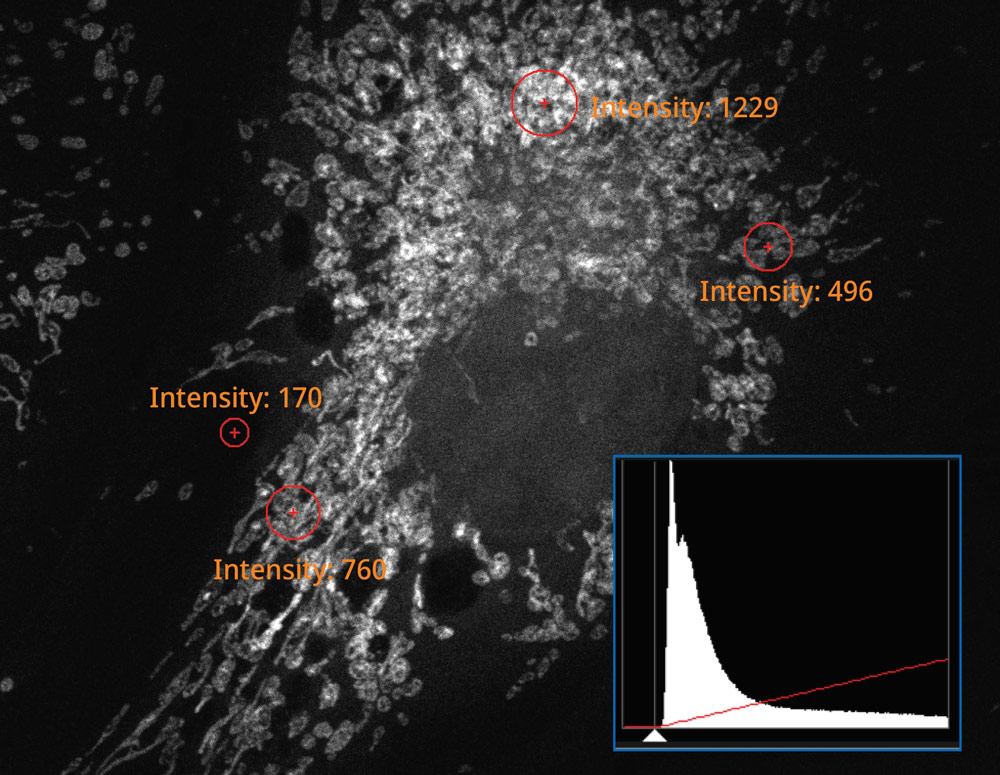
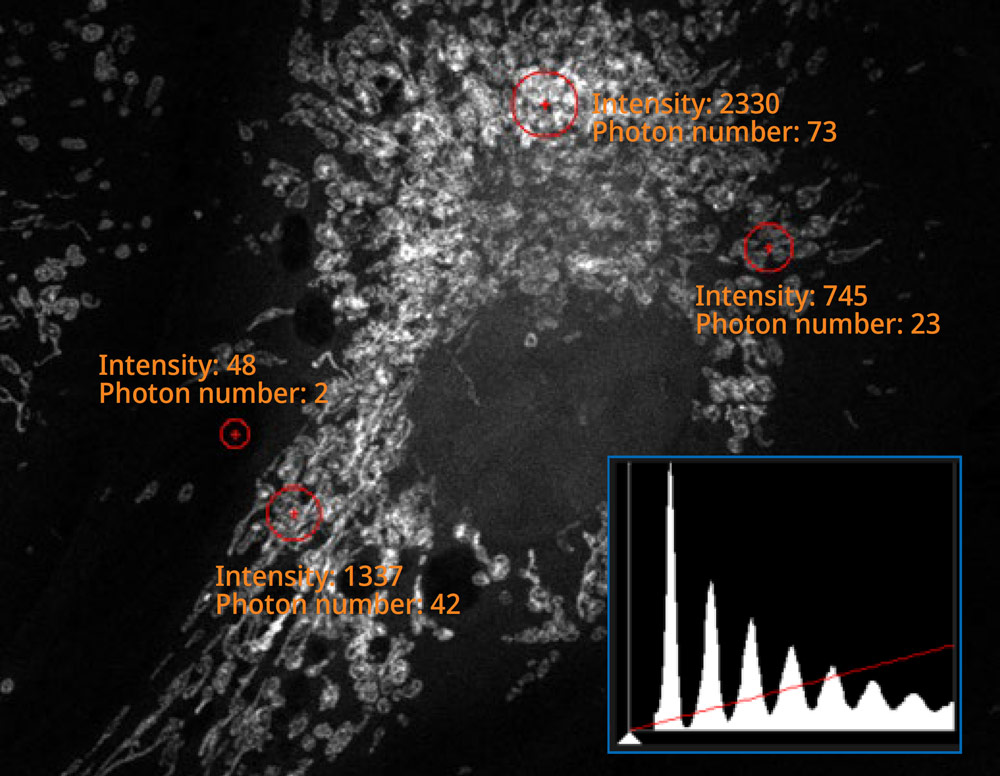
The histogram on the image captured using the SilVIR detector shows a discrete pattern where the intensity can be converted to the photon number. The detector’s fluorescence intensity can be quantified as the photon number, and the background level is extremely low.
More Information from Your Confocal Images
The system’s updated TruSpectral technology combined with high sensitivity SilVIR detectors enable you to see more by making it possible to multiplex up to six channels simultaneously
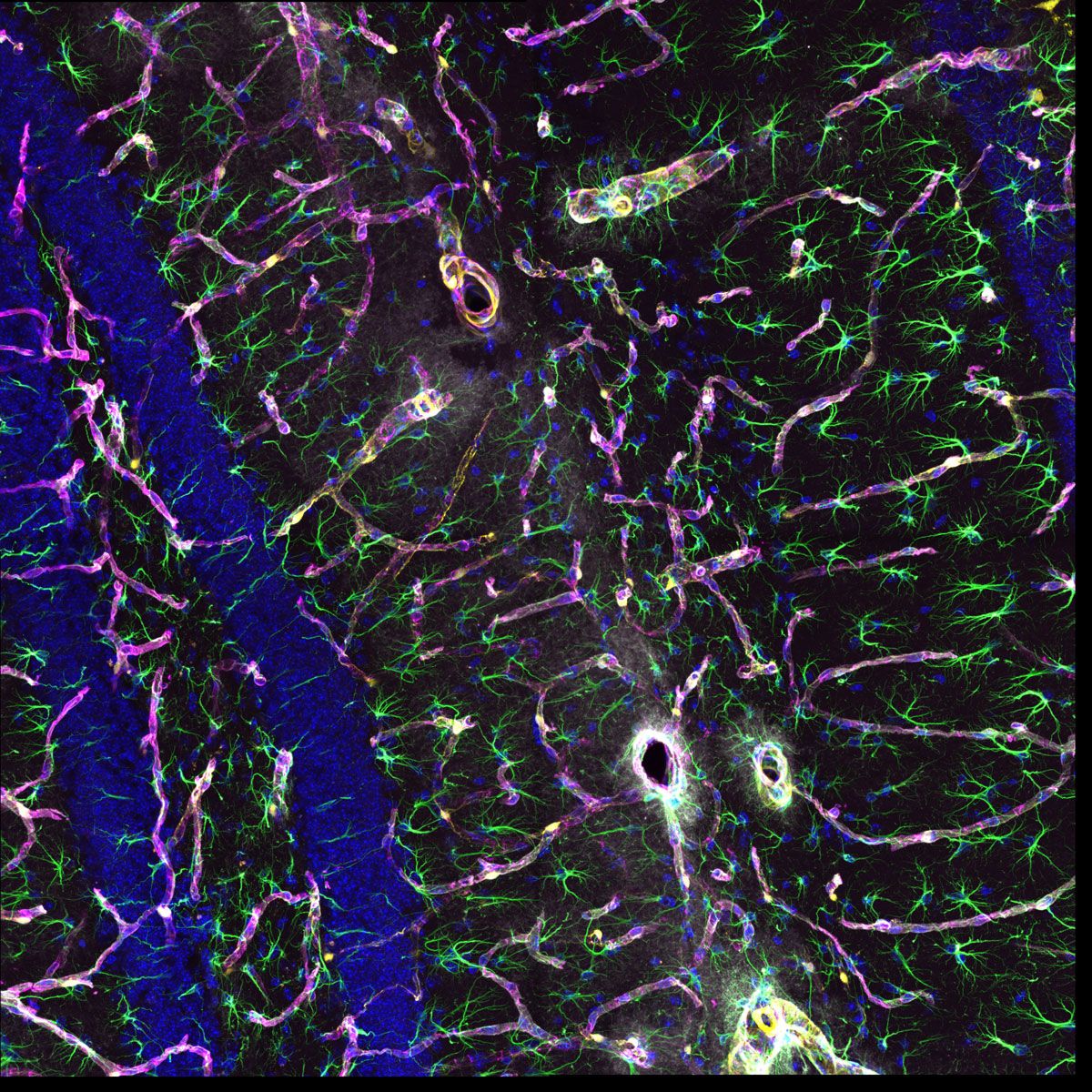
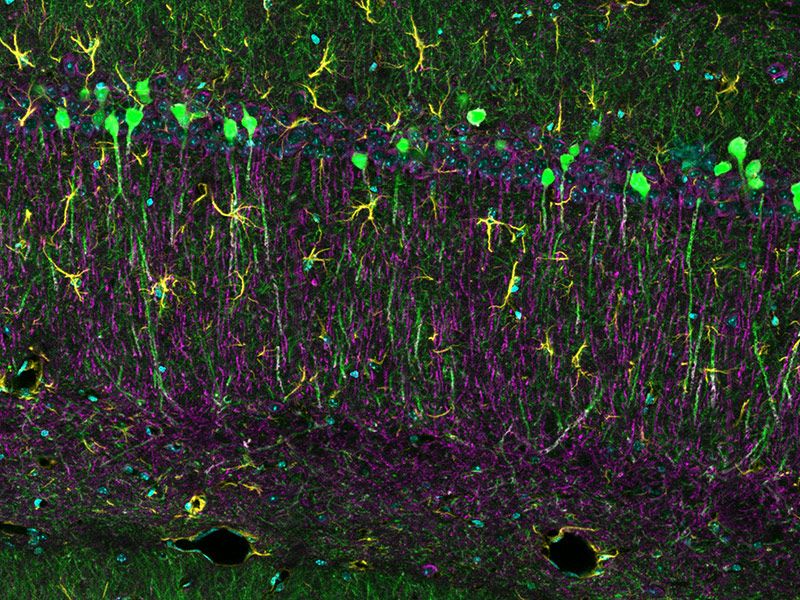
Sample courtesy of: Hiroshi Hama and Atsushi Miyawaki, Cell Function Dynamics, RIKEN CBS.
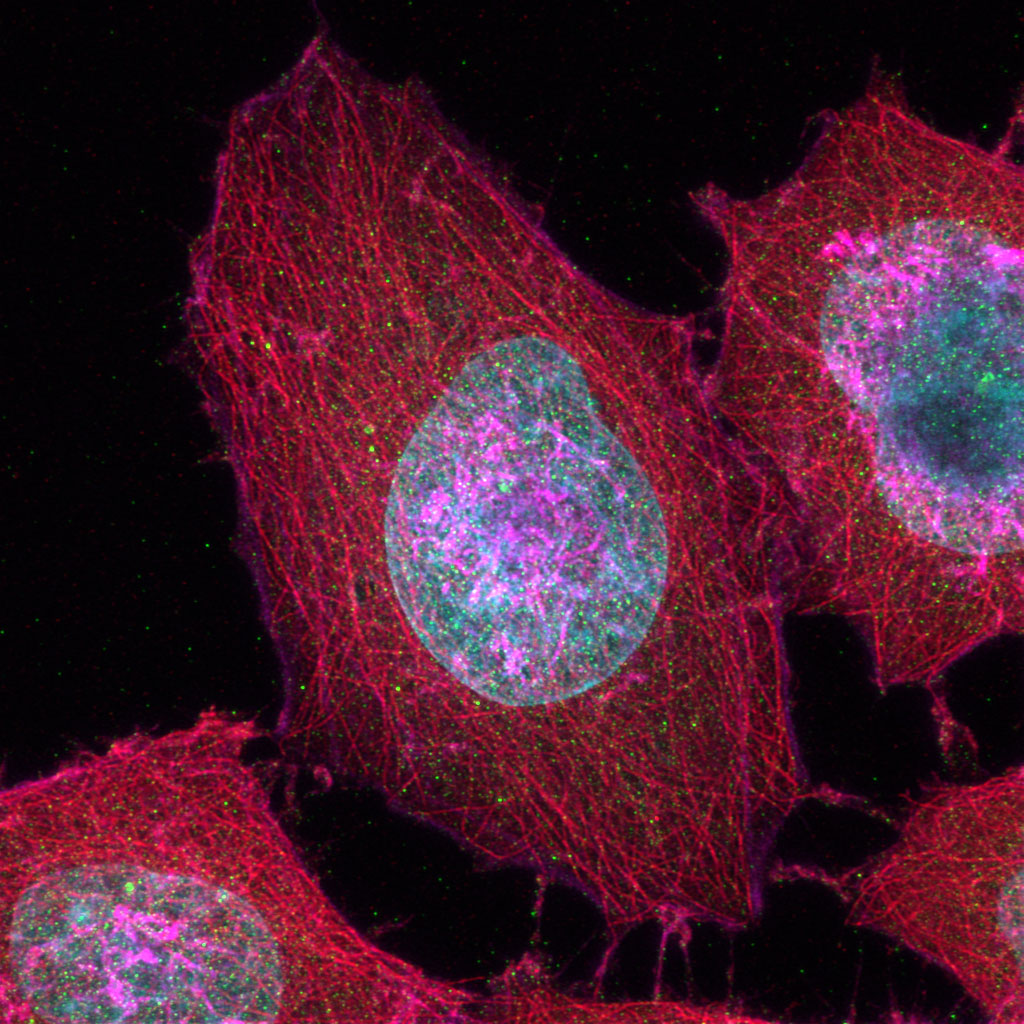
Sample Courtesy of: Sample preparation Alexia Ferrand; sample acquisition Sara R. Roig and Alexia Ferrand. Imaging Core Facility, Biozentrum, University of Basel.
Flexible Macro to Micro Imaging
The macro-to-micro workflow enables you to easily observe the target sample from the macro level—whole body or tissue—down to the cell or subcellular level.
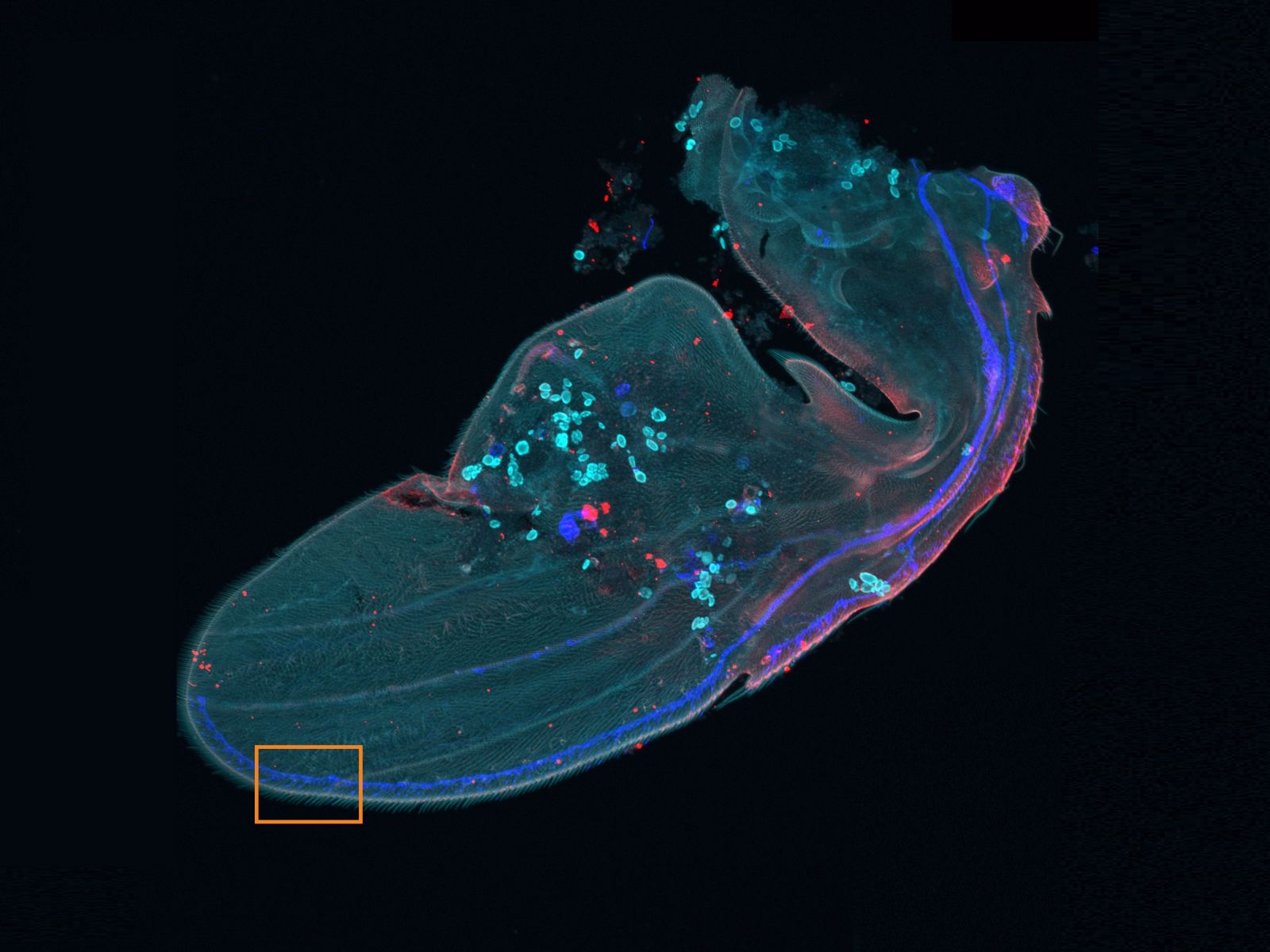
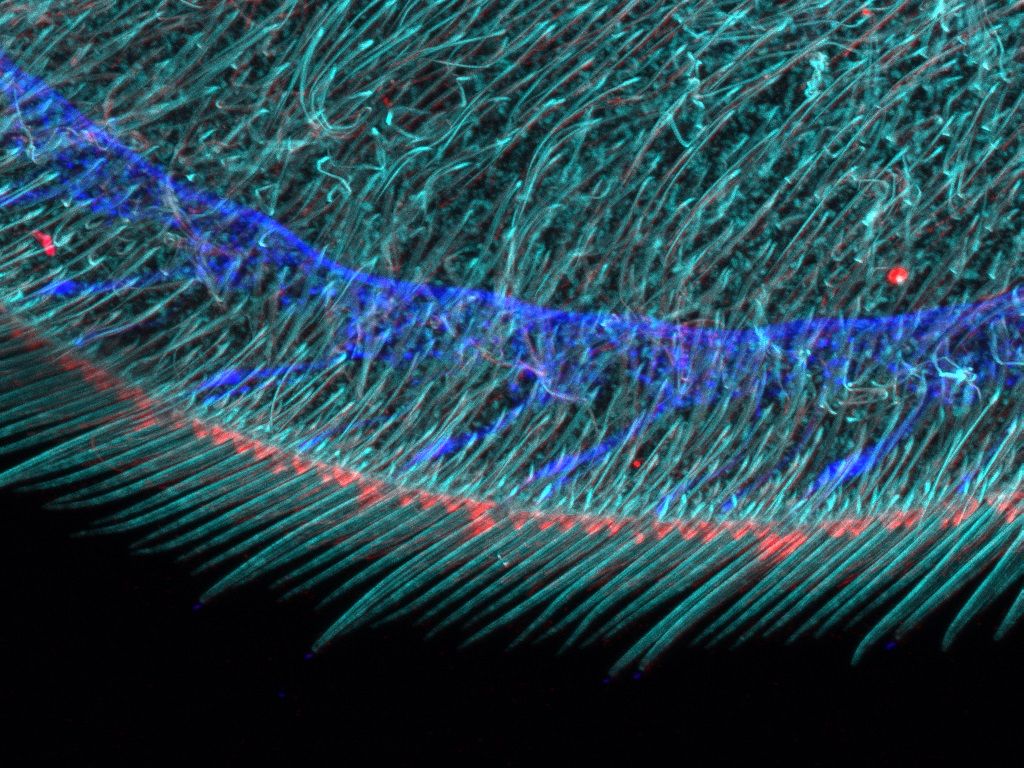
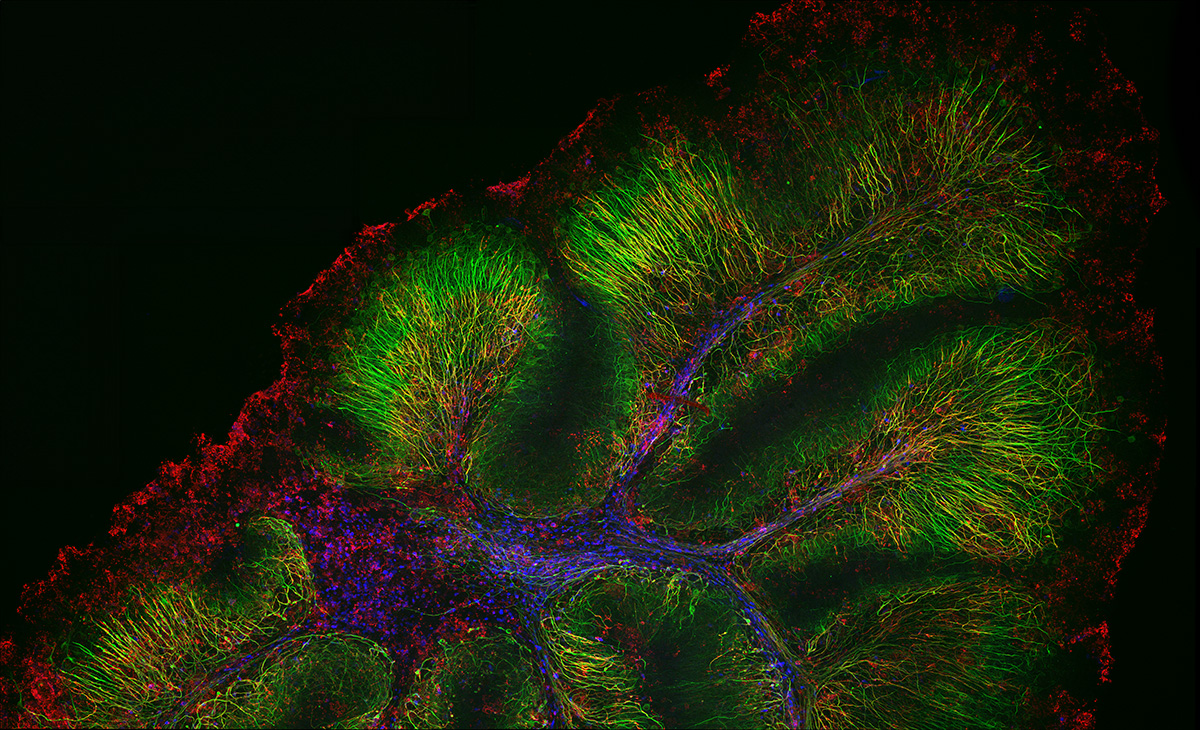
Overview and edge images of a Drosophila wing (42-hour pupation). Stained with phalloidin (AlexaFluor 405, F-actin, Cyan), anti-phosphotyrosine antibody (AlexaFluor 555, cell surface, red), and anti-HRP antibody (AlexaFluor 647, axon, blue). Sample courtesy of: Sun Zhengkuan, Shigeo Hayashi, Laboratory for Morphogenetic Signaling, RIKEN Center for Biosystems Dynamics Research, Japan.
Gentler High-Speed Time-Lapse Confocal Imaging
Time-lapse imaging is easier with smart features:
- Capture every moment of live cell dynamics: resonant scanner can acquire high-resolution images over a wide area
- Minimal phototoxicity: the scanner’s short pixel dwell time reduces the time the focused laser beam rests on a single spot
- Better signal-to-noise ratio: the SilVIR detector’s high sensitivity produces higher quality images at higher speeds
- Greater precision: rolling average processing maintains qualifications and time resolution
Reproducible Image Data Between Users and Systems
The SilVIR detector has less sensitivity loss over time compared to previous-generation detector technologies. With our laser power monitor (LPM) and TruFocus™ Z-drift compensator, achieve reproducible images under consistent conditions for better reproducibility. Different users on different days can acquire the same precise images using the same settings. Even the images acquired by different FV4000 microscopes can be compared and discussed using the same photon number intensity scale.
Microscope Support and Service You Can Count On
We designed the FV4000 system to be easy to maintain:
- Semiconductor-based SilVIR detector is stable and durable
- Laser power monitor continuously checks the illumination condition and makes adjustments to maintain consistent laser power
- System admin can view log files to keep track of the service maintenance schedule
We stand behind our products with a commitment to fast service and technical support. We offer various support plans to keep your microscope running at peak performance at a predictable cost as well as remote support options, so you don’t need to wait for an engineer or specialist to visit if you’re having an issue.

See Further with NIR-Enabled Confocal Microscopy
The system’s enhanced technologies provided expanded multiplexing to see more in one image.
NIR imaging offers greater multiplexing capabilities by extending the excitation (λ_Ex) and detection (λ_Em) spectral profile of the FV4000 system. This enables additional dyes to be used to help minimize emission signal overlapping.
- Multiplex up to six channels with updated TruSpectral™ technology and our high sensitivity SilVIR™ detectors
- High-efficiency volume phase hologram (VPH) grating and slit can detect an industry-leading 400 nm to 900 nm wavelength range* with a minimum 1 nm step
- Expand your fluorochrome choices with up to six channels of broadband or red-shifted detectors to minimize damage and reduce autofluorescence
- Modular laser combiners allow up to 10 laser lines from 405 nm to 785 nm in parallel
*As of March 2023.
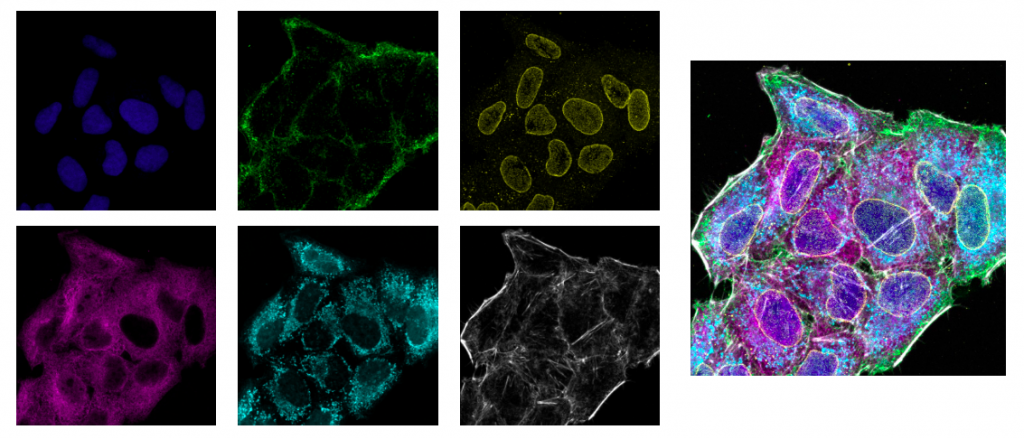
Cell nuclei (DAPI; blue), cell membran (AF488; green), nuclear pore (AF561; yellow),
microtubule (Qdot605; magenta), mitochondria (MitoTracker DeepRed; cyan), actin (AF750 phalloidin; gray).
High-Quality Optics for Efficient NIR Fluorescence Imaging
The FV4000 system’s optical elements have a high transmission from 400 nm to 1300 nm, including the galvanometer and resonant scanner, which are coated in silver rather than aluminum.
Our award-winning X Line™ objectives are corrected for chromatic aberrations between 400–1000 nm. They also have a higher numerical aperture, excellent flatness, and very high transmittance from UV to NIR, increasing the multiplexing capabilities.
For improved colocalization reliability, our specialized A Line™ (PLAPON60XOSC2) oil immersion objective (ne~1.40) significantly minimizes chromatic aberration for strict colocalization analysis.

High-Quality Confocal Images at High Speed
A unique combination of advanced technologies delivers high-quality images faster than conventional laser scanning microscope systems.
- High-resolution images at high speed: 1K × 1K resonant scanner at FN20 with 0.033 µs per pixel and the SilVIR detector enable you to rapidly acquire images with minimal noise
- Exceptional quality macro images: quickly acquire stitched macro images with outstanding quality to maximize your time and research potential
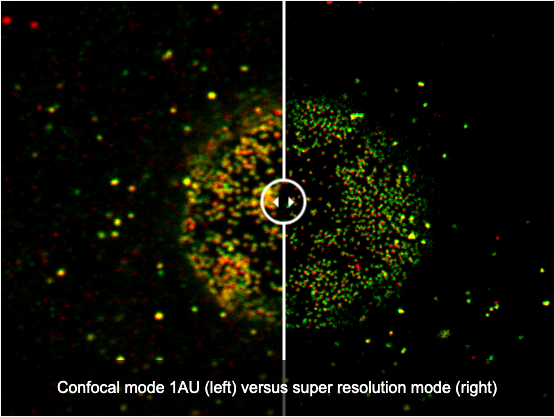
Simple, Precise Super Resolution Imaging
Capture super resolution images using the FV4000 microscope with no dedicated hardware.
- Easily observe subcellular structures using our A Line HR objectives and our super resolution software (FV-OSR)
- FV-OSR automatically optimizes the confocal aperture to detect high-frequency components and enhance their contrast to achieve 120 nm resolution
- Achieve super resolution images 8x faster than previous-generation systems thanks to the SilVIR detector and on-the-fly processing
High-Resolution 3D Images in Thick Samples
When imaging thicker samples, the FV4000 microscope enables you to capture high-resolution, 3D images.
- Take advantage of NIR’s longer wavelength to penetrate deeper into tissue thanks to the SilVIR detector’s wide dynamic range and sensitivity
- Image deeper with less scattering and absorption by taking advantage of the fact that light-scattering compounds—like melanin and heme—absorb less light between 700–1500 nm
- Image significantly deeper than what’s possible with visible lasers thanks to 685 nm, 730 nm, and 785 diode lasers on the FV4000
- High NA silicone objectives minimize spherical aberration and silicone oil doesn’t dry out, both advantageous for time-lapse imaging
- Improve overall image quality and Z resolution using TruSight™ deconvolution for stunning 3D images of thick samples
- Enjoy a seamless workflow from acquisition to publication with specialized cellSens™ software algorithms
Precise Dynamics of Live Cells with Less Damage
Typically, using longer wavelengths for fluorescence excitation for shorter periods of time is better for overall sample health. Using less phototoxic light means you can image for longer periods, enabling you to obtain more consistent and reproducible data from live cell imaging experiments.
The FV4000 system not only provides gentle time-lapse imaging via the 685 nm, 730 nm, and 785 nm lasers, but it also features a dedicated TruFocus Red Z-drift compensator to maintain the focus position. This upgraded TruFocus Red unit supports a larger range of wavelengths and is compatible with a wide range of objectives, including our high-performing X Line™ and A Line™ series.
Clear Images at Depth
Use our silicone immersion objectives with the FV4000 microscope and achieve clear images of features and structures deep within your sample. Silicone oil has a refractive index close to that of live cells or tissue, greatly reducing the spherical aberration as compared to air, water, or other oils. With less aberration, you can achieve clearer images of your sample at depth. And silicone immersion oil does not dry out at 37 ℃ (98.6 °F), making it effective for long-term time-lapse imaging.
| Scanner | Galvanometer scanner (normal imaging) | 64 × 64 to 4096 × 4096 pixels, 1 μs/pixel–1000 μs/pixel |
| Resonant scanner scanner (high-speed imaging) |
512 × 512 pixels, 1024 × 1024 pixels | |
| Field number (FN) | 20 | |
| Spectral confocal detector | Detector | SilVIR detector (cooled SiPM, broadband type/red-shifted type) |
| Maximum channels | Six channels | |
| Spectral method | VPH, detectable wavelength range 400 nm–900 nm | |
| Laser | VIS laser | 405 nm, 445 nm, 488 nm, 514 nm, 561 nm, 594 nm, 640 nm |
| NIR laser | 685 nm, 730 nm, 785 nm | |
| Laser power monitor | Built in | |
| Image | High dynamic range photon counting (1G cps, 16-bit) | |
For more information on Evident-Olympus products, click here.